3022A
Product Specifications
500ml
Comprehensive physicochemical and microbiological testing, strictly following pharmacopoeial standards.
Standard execution
Keywords:
3022A
 Product Inquiry
Product Inquiry
3022A
|
|
|
|
Product Information
| Product Catalog Number | 3022A | Shipping Method | Dry ice, cold chain |
| Product Specifications | 500ml, 50ml*10 | Endotoxin Content | ≤1EU/ml |
| Blood origin | China | Hemoglobin Content | ≤200mg/L |
| Storage Conditions | (-10~-30)℃ | Protein Content | 30-45g/L |
| Applicable Types | Tumor cells Some nerve cells Some primary cells Some stem cells |
Biosafety Monitoring | No bacteria, fungi No mycoplasma No viral contamination |
Application Cases
MSC cells
Culture Methods and Materials
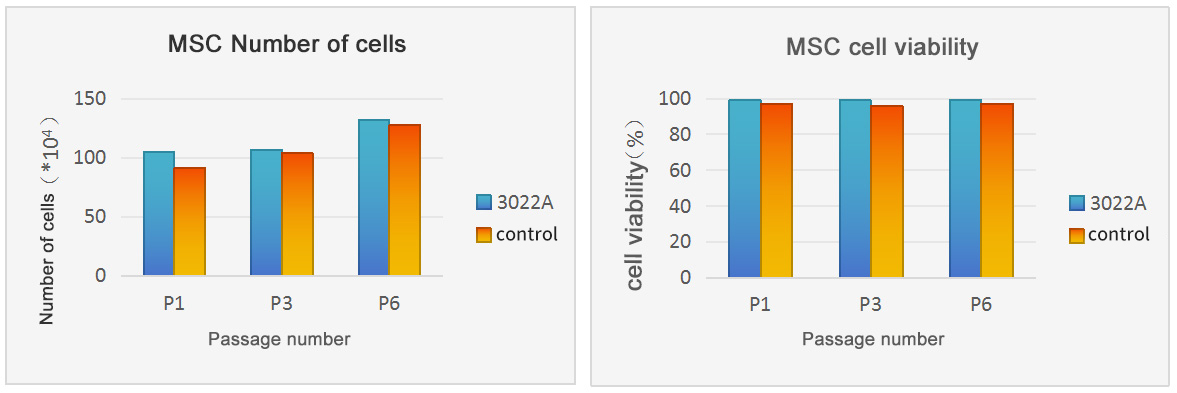
Passage method: Cells are inoculated at 1*105 per generation cells, 72h, continuous culture for 6 generations
Culture system: α-MEM+10%FBS+10ng/ml bFGF, 6-well plate
Culture conditions: 37℃, 5% CO2
Control serum: G brand Australian serum
Cell Images
|
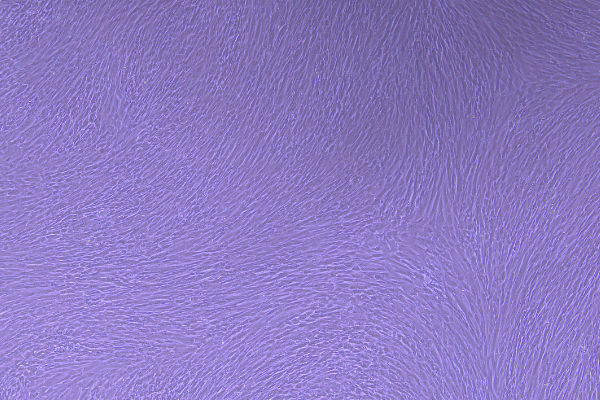
3022A (P3, 4×, 72h) |
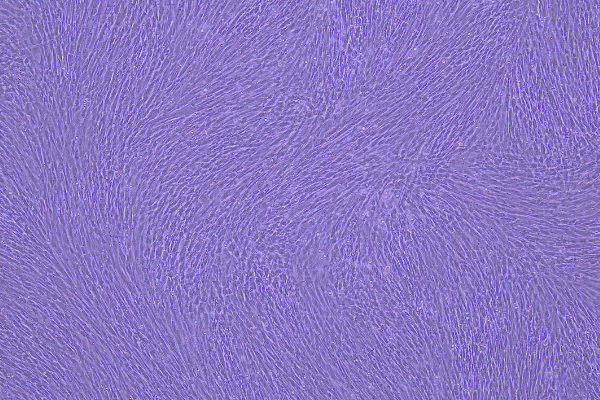
Control (P3, 4×, 72h) |
3022A (P6, 4×, 72h) |
Control (P6, 4×, 72h) |
Cell Counting
Online Message
If you have any suggestions, please leave a message or email us, and we will reply to you within one working day of receiving your message or email.